
Contact our advisors at Modirfa to get a free consultation for obtaining the ISO 13485 standard
02188764867 – 02188761795
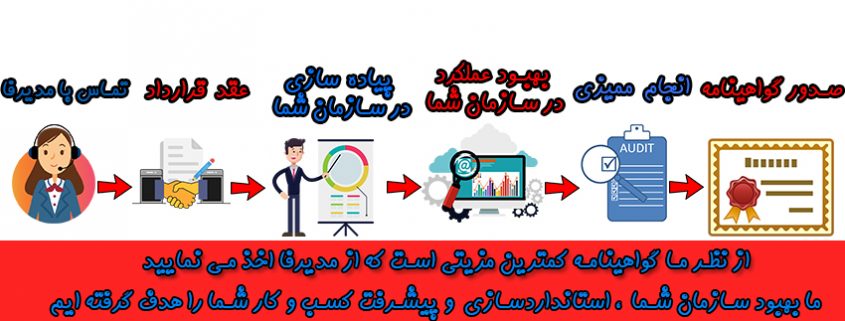
Consulting for certification ISO13485:2016 standard
Modirfa is proud that after changing the ISO13485:2016 standard version, it has succeeded in implementing the ISO 13485 consultation and implementing this standard in a significant number of Iranian medical equipment manufacturing companies.
Obtaining ISO13485:2016 standard certificate
Obtaining the ISO13485:2016 standard certificate is a requirement of the General Directorate of Medical Equipment of the Islamic Republic of Iran to obtain a license to operate under the supervision of IMED, therefore, all manufacturers and importers of medical equipment must implement the ISO13485:2016 standard consultation and obtain the ISO 13485 certificate. ISO13485:2016 standard certificate should be obtained from the list of CB certification companies trusted by IMED. This list is constantly changing, so it is recommended to consult with our advisors in the management of ISO13485:2016 before choosing a company that certifies the ISO13485:2016 standard in order to save time and money significantly.
Benefits of ISO13485:2016 standard consulting
As one of the oldest and most experienced ISO 13485 consulting companies in Iran, Madirfa is proud to provide ISO13485:2016 standard consulting for many medical equipment manufacturers in Iran, ready to cooperate and provide ISO13485 consulting and obtain a valid certificate accepted by the department. Dear all, medical equipment is provided as soon as possible and with the best management consulting methods.
One of the advantages of consulting the ISO13485:2016 standard in the organization is the implementation of the requirements of the quality management system in the organization because this standard overlaps a lot with ISO 9001 standard The 2015 version has a great help in improving the quality management system in the organization as well as increasing the level of customer satisfaction. The new version of ISO13485 with the 2016 version provides suitable solutions in the direction of quality management, which is more useful for the organization than the framed and installed certificate in the organization.
Contact our advisors at Modirfa to get a free consultation for obtaining the ISO 13485 standard
02188764867 –02188761795
Obtaining the ISO 13485 certificate is a necessary condition for obtaining a license to produce span, and operate from the General Administration of Jumping Equipment of the Islamic Republic of Iran..
Beware of unlicensed and fraudulent organizations.
Be sure that if an organization gives you an ISO certificate without implementing it in your organization and conducting an audit, it must be a fraud.
The cost of obtaining the ISO13485:2016 standard
Obtaining the ISO13485:2016 standard includes two major parts. The first part is related to consulting costs and the second part is related to audit and registration and issuance costs. Audit costs depend on the type of CB (certifying company) chosen to receive the certificate and depend on many parameters, the most important of which are the number of personnel, the number of processes, the scope of work and the number of branches of the organization (head office , factory, sales branches, etc.) mentioned. Different CBs charge their own fees depending on their credit rating.
You can implement the standard requirements internally and using your knowledge and experience in the organization. You can also use the ISO13485:2016 standard consulting of the modirfa team to speed up the implementation of the requirements and also use the experiences of the modirfa team. use You can contact us to get more information about the ISO13485:2016 standard consulting fee.
Procedures for obtaining the ISO13485:2016 standard
In order to receive the ISO13485 standard, you must first implement the requirements of the standard in your organization and also have at least one technical person in charge based on the type of product and the degree or class of risk that you provide. After implementing the requirements and preparing for the audit, the CB certification companyself specify and conclude an “audit, registration and certification” contract with them.
A very important point is the choice of CB. When choosing CB, you must choose one of the companies approved by the General Department of Medical Equipment IMED. The top organization in the field of medical equipment in Iran is the General Directorate of Medical Equipment. So you should choose your certification company based on the approved list of this organization. This list is always changing and the advisors of Modirfa are by your side so that you don’t have any worries in choosing the certification company approved by IMED.
For advice on establishing ISO13485 medical equipment industry quality management system, contact our colleagues at Modirfa and receive definitive answers to all your questions.
02188761795 – 02188738902

